Sarepta Surges Over 14% as FDA Lifts Shipping Ban; Wall Street Anticipates Doubling in Stock Price
TradingKey - Sarepta Therapeutics (SRPT.US) announced on Monday, July 28, that it has resumed shipments of its gene therapy Elevidys in the U.S., following an authorization from the U.S. Food and Drug Administration (FDA). The company's stock spiked over 20% on Tuesday, closing up 14.21% at $15.83, with gains continuing in after-hours trading.
Elevidys is the only FDA-approved gene therapy for the rare Duchenne muscular dystrophy (DMD). However, safety concerns arose earlier this year after two patient deaths were linked to the therapy.
On July 18, the FDA halted Elevidys shipments to investigate these deaths, causing Sarepta's stock to plummet nearly 40%. It wasn't until this Monday that the FDA found no direct link between the therapy and the incidents, allowing the shipping ban to be lifted.
Despite Tuesday's surge, Sarepta's stock has fallen 86.98% this year due to safety concerns.
Analysts remain optimistic about the company's future. William Blair's Sami Corwin expressed confidence in the resumed shipments, suggesting it nearly eliminates the risk of Elevidys being pulled from the market. Jefferies analyst Andrew Tsai maintains a "buy" rating, confident in the company's ability to relaunch its product, and is currently monitoring sales performance.
In contrast, some experts remain cautious. Leerink Partners analyst Joseph Schwartz described the development as positive but intends to observe market demand trends for Elevidys. Analysts are also monitoring how this impacts the company’s earnings, given Sarepta's $1.5 billion convertible debt due in 2027. Strong sales of Elevidys are crucial to achieving profitability.
According to TradingKey data, the consensus rating among analysts is "hold" with a price target of $30.614, indicating potential upside of over 100%.
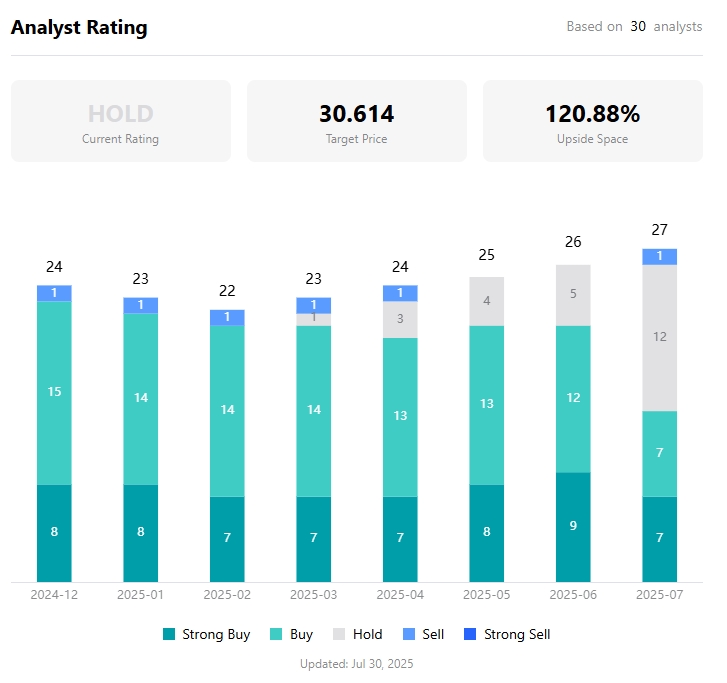
Analyst rating, Source: TradingKey



